Updated at 4:16 a.m. on Oct. 21 to add RDIF's statement.
The European Union’s drug regulator will not approve Russia’s Sputnik V coronavirus vaccine until at least the first months of 2022, Reuters reported Thursday, citing unnamed sources.
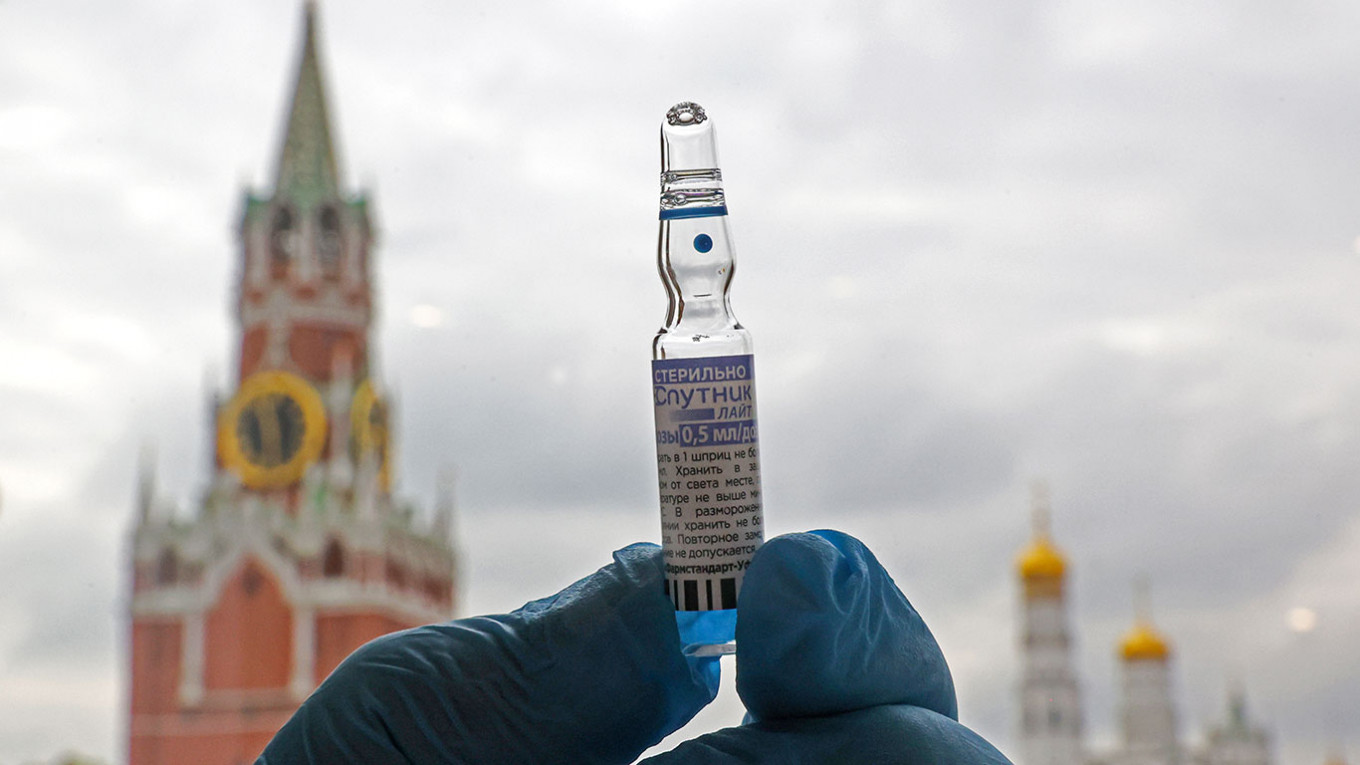
Russia has been eager for international authorization of Sputnik V, which Moscow touts as the world’s first Covid-19 vaccine. EU and World Health Organization (WHO) approval of Sputnik V would allow the shot’s manufacturers to compete with western drugmakers Pfizer and Moderna and ease international travel for Sputnik-vaccinated Russians.
The European Medicines Agency (EMA) “may well decide in the first quarter of next year” whether to approve Sputnik V, Reuters quoted an unnamed source with knowledge of the matter as saying.
But that hinges on whether the Russian manufacturer provides the still-missing data needed for the EMA’s ongoing review by the end of November.
“An EMA decision by the end of the year is now absolutely impossible,” the source was quoted as saying.
The EMA said in an email that Sputnik V remains under so-called rolling review until a formal “marketing authorization application has been submitted to the agency,” Reuters reported.
The news agency’s source said the EMA had asked the Russian manufacturer for a “more complete dossier” on the production of Sputnik V’s active ingredient and the bottling of the finished product.
“When they have this dossier they will also be able to understand where to ask for inspections,” the source said.
The EMA’s review has been delayed because the Gamaleya Institute, Sputnik V’s maker, does not have experience dealing with an international drug regulator and has changed production sites for EU-bound vaccine doses several times, Reuters cited two sources as saying.
The Russian Direct Investment Fund (RDIF), which markets Sputnik V internationally, decried what it said was an increase in “attacks in the press based on wrong and misleading information from anonymous sources” after studies showing Western vaccines’ lower efficacy against the Delta variant compared with Sputnik V.
“Instead of attacking Sputnik, we urge Western mRNA vaccine producers to consider using the one-component Sputnik Light as a booster to help them achieve stronger immunity that lasts longer,” it said in a statement Thursday.
RDIF did not directly confirm or deny that the EMA’s approval process has been delayed until early 2022.
The WHO, which is also conducting a separate review of Sputnik V, said earlier this month that problems behind its suspension of the vaccine’s approval process had been resolved. The RDIF had said that a group of WHO inspectors were due to visit Russia “shortly.”
International authorization of Sputnik V may run up against a supply crunch as all but one Russian region have mandated vaccinations for certain categories of the population to battle the country’s deadliest wave of the pandemic.
A Message from The Moscow Times:
Dear readers,
We are facing unprecedented challenges. Russia's Prosecutor General's Office has designated The Moscow Times as an "undesirable" organization, criminalizing our work and putting our staff at risk of prosecution. This follows our earlier unjust labeling as a "foreign agent."
These actions are direct attempts to silence independent journalism in Russia. The authorities claim our work "discredits the decisions of the Russian leadership." We see things differently: we strive to provide accurate, unbiased reporting on Russia.
We, the journalists of The Moscow Times, refuse to be silenced. But to continue our work, we need your help.
Your support, no matter how small, makes a world of difference. If you can, please support us monthly starting from just $2. It's quick to set up, and every contribution makes a significant impact.
By supporting The Moscow Times, you're defending open, independent journalism in the face of repression. Thank you for standing with us.
Remind me later.